This is a working model to show that salt water conducts electricity. Pure water is a poor conductor, but when salt (NaCl) is added, it dissociates into Na⁺ and Cl⁻ ions, which help carry electric current.
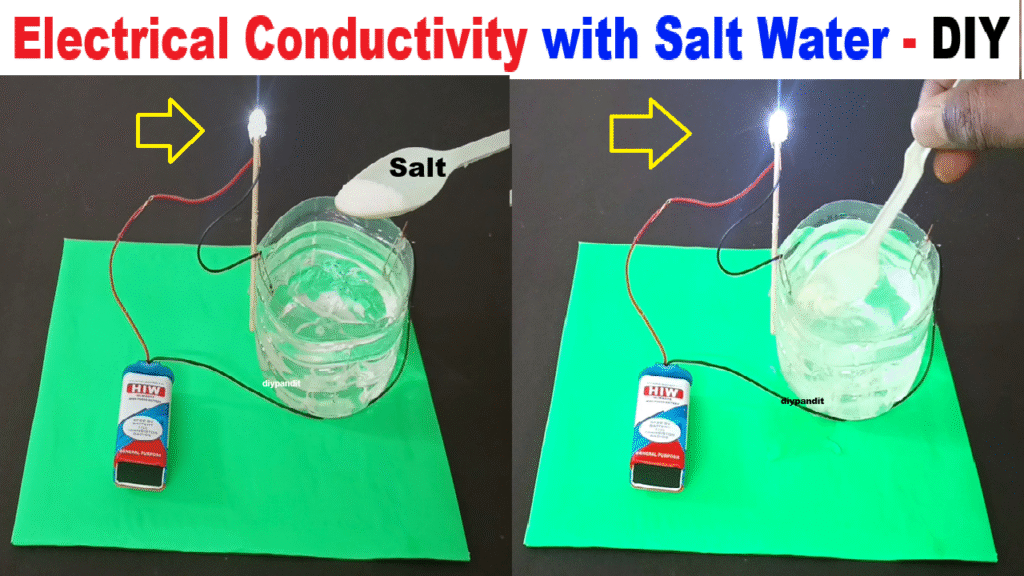
This is why the LED/buzzer only works when salt is mixed with water. It demonstrates the concept of electrolytes and ionic conduction in liquids.
Materials Needed
- Half plastic bottle (cut a bottle horizontally)
- 9V battery + battery clip
- 2 safety pins (or metal nails)
- Salt
- Water
- 1 LED or buzzer (to show conductivity)
- Wires with alligator clips or tape
- Small resistor (optional, to protect LED)
How to Make It Salt water conducts electricity experiments
Step 1: Prepare the Conductivity Container
- Cut a plastic bottle in half — use the bottom half as your container.
- Fill it halfway with water and mix in 2–3 tablespoons of salt. Stir well until dissolved.
Step 2: Insert Electrodes
- Pierce the sides of the plastic bottle with two safety pins facing each other but not touching.
- These will act as electrodes.
Step 3: Wiring the Circuit
- Connect:
- One end of the 9V battery to one safety pin (via wire or alligator clip).
- The other safety pin to the positive leg of an LED or one side of a buzzer.
- The other leg of LED or buzzer goes back to the battery’s other terminal.
In short:
Battery (+) → Safety Pin → Salt Water → Safety Pin → LED/buzzer → Battery (–)
Step 4: Test the Setup
- With plain water, the LED/buzzer should not work.
- Now add salt and stir — the LED will glow or buzzer will beep, proving salt water conducts electricity.

