INTRODUCTION
In this topic, we are going to show you the experiment on electrolysis of water for your science project or exhibitions(also called an electrolysis working model)
Water Components & splitting
Water is H₂O and most common chemical compound found on earth.
Water helps us to survive and the human body is mostly composed of it.
Water is made up of mainly two hydrogen atoms bound to one oxygen atom.
Water is less dense when solid (ice) in comparison to liquid which has major consequences for the planet and its climate.
It is good at storing heat and stays remains in a liquid state over a range of temperatures.
Electrolysis of water(Process explanation)
Water electrolysis is a chemical process in which water is separated into its component parts, hydrogen and oxygen, using an electric current.
This process can be used to produce hydrogen gas, which has a variety of practical applications, including use as a fuel and in the production of chemicals.
We can breakdown water into Hydrogen and Oxygen components with two regular pencils and a 9V battery.
During the process bubbles of gas generates when water starts to boil.
If you are interested in conducting a water electrolysis science project, there are a few key steps you can follow:
- Gather your materials: You will need a power source (such as a battery), electrodes (such as copper wire or strips of copper or pencil), and water. You will also need a device to measure the volume of gas produced, such as a gas pressure sensor or a gas syringe.
- Set up your experiment: Connect the electrodes to the power source(battery), and place them in a plastic container of water. Make sure that the pencil electrodes are not touching each other.
- Turn on the battery power: Turn on the power source, and observe the reaction. You should see the evolution of hydrogen gas at the negative electrode (cathode) and oxygen gas at the positive electrode (anode).
- Measure the volume of gas produced: Use your measuring device to measure the volume of hydrogen and oxygen gas produced.
- Analyze your results: Calculate the volume of hydrogen and oxygen produced, and compare your results to the predicted values based on the stoichiometry of the reaction. Consider any deviations from the expected results, and try to identify possible sources of error.
Electrolysis of water Experiments
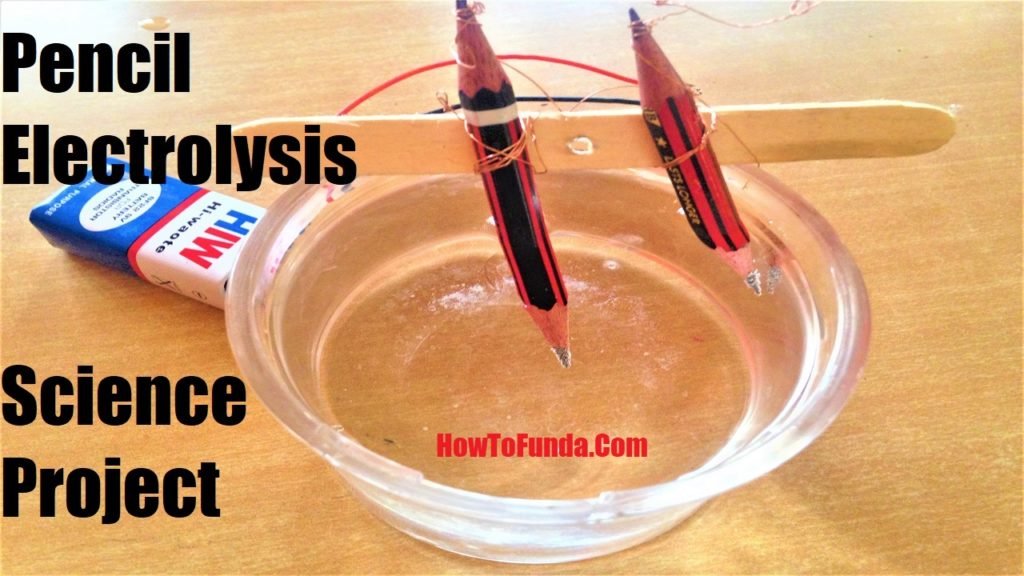
Immerse free pencils ends into the water and ensure that tips don’t touch each other. Water becomes part of the circuit in this experiment
Electrons flow down from one pencil through water and into another pencil.
This helps in creating a circuit and electrons flow freely around it.
This process quickly creates bubbles at the pencil tips in the water which shows that it working properly.
Electrons energy in the circuit is quite enough to break water into Oxygen and Hydrogen ions.
These ions interns flow freely through the water to oppositely charged pencil tips resulting in an electric circuit.
This is called electrolysis and also called has splitting water experiment
Positively charged hydrogen ions meet at negative pencil and result in hydrogen gas (H₂) that bubbles to the surface.
On the other hand, positive pencil tip draws negative Oxygen ions which results in the formation of O₂.
Uses of electrolysis of Water
As part of simple electrolysis of water, The hydrogen is produced that can be used for the production of specialty chemicals or various other small-scale applications. Oxygen is also produced that can be used in the International Space Station.
Step by Step electrolysis of water experiment video
In this video, we will show you the steps by step electrolysis of water experiment video at home by taking help freely from your parents.
Materials Used for electrolysis of water experiment video
- Plastic container
- Two sharpened (both sides) pencils
- Copper wire
- Ice cream stick
- Battery
- Water(regular or salt water also fine)
Questions & Answers on electrolysis of water experiment
What is water electrolysis used for?
Electrolysis is mainly used to produce hydrogen through the process of electrolysis.
What happens during the electrolysis of water?
During electrolysis of water, it gets decomposed by passing an electric current through it which result in an oxidation-reduction reaction.
What is the apparatus used for the chemistry electrolysis of water experiment called?
Brownlee electrolysis apparatus.
What does electrolysis of water do?
It helps in the decomposition of water into hydrogen gas and oxygen due to the passing of electricity. It helps to make hydrogen gas and breathable oxygen.
Can pure water undergo electrolysis?
No, as pure water does not contain free-flowing ions to conduct electricity.
Conclusion of electrolysis of water experiment
Pencil electrolysis experiment helps the students or kids in understanding how we can generate oxygen and hydrogen from water through the process of electrolysis by passing an electric current through water.

