Introduction:
Overview: Acid rain is a type of precipitation that is more acidic than normal rain due to the presence of certain pollutants in the atmosphere. These pollutants, primarily sulfur dioxide (SO2) and nitrogen oxides (NOx), react with water vapor to form sulfuric and nitric acids. When this acidic moisture falls to the ground, it can harm plants, aquatic life, infrastructure, and even human health.
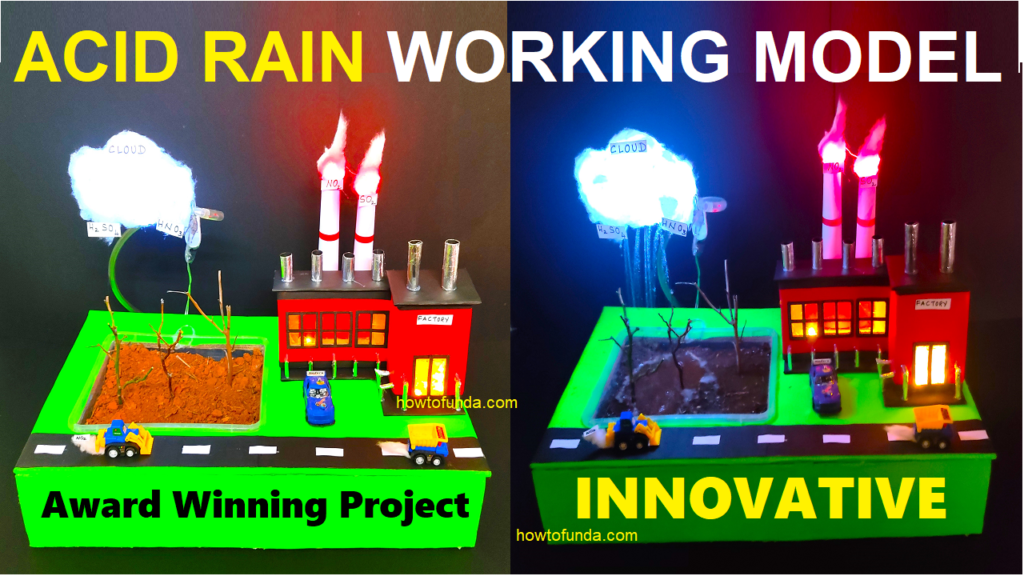
Components of the Acid Rain Working Model:
Let’s first look at the components of our acid rain working model:
- Pollution Sources: A model factory and car, representing sources of sulfur dioxide and nitrogen oxides.
- Water Vapor: A spray bottle representing water vapor in the atmosphere.
- Acid Formation: A container with vinegar (acetic acid) to simulate the formation of acid.
- Precipitation: A way to simulate rain, like a small watering can.
- Effect on Environment: Various materials like plants, soil, and metals to show the effects of acid rain.
Demonstration of Acid Rain Working model :
- Pollution Emission:
- First, we’ll show how pollutants are released into the atmosphere. Factories and vehicles emit sulfur dioxide and nitrogen oxides.
- Show in the working model factory and car. Use a small smoke machine or dry ice to represent emissions if available.
- Acid Formation:
- These pollutants rise into the atmosphere and mix with water vapor to form acids. In our model, we’ll use vinegar to represent this process.
- Spray water from the spray bottle to simulate water vapor. Add a few drops of vinegar to simulate the formation of acidic compounds.
- Acidic Precipitation:
- When it rains, these acids come down with the precipitation, forming acid rain.
- Use the watering can to let the acidified water fall on the plants, soil, and metals.
- Effects of Acid Rain:
- Now, let’s observe the effects of acid rain on different materials.
- Show how the :
- Plants: Point out how plants might wilt or show signs of damage.
- Soil: Explain how acid rain can leach important nutrients from the soil, affecting plant growth.
- Metals: Show how metals can corrode faster in the presence of acid rain.
Real-World Impact with acid rain:
- Environmental Damage: Acid rain can harm forests, lakes, and streams. It can cause trees to lose their leaves and fish to die in water bodies.
- Human Structures: Buildings and monuments, especially those made of limestone and marble, can be severely damaged by acid rain.
- Health Impacts: While acid rain itself doesn’t harm humans directly, the pollutants that cause it can lead to respiratory problems.
Conclusion:
To summarize, our model demonstrates how pollutants from factories and cars cause acid rain, which then harms the environment and infrastructure. Acid rain is a serious issue that results from air pollution and has widespread impacts. Remember, reducing air pollution by using cleaner energy sources, conserving energy, and supporting environmental regulations can help reduce acid rain.

