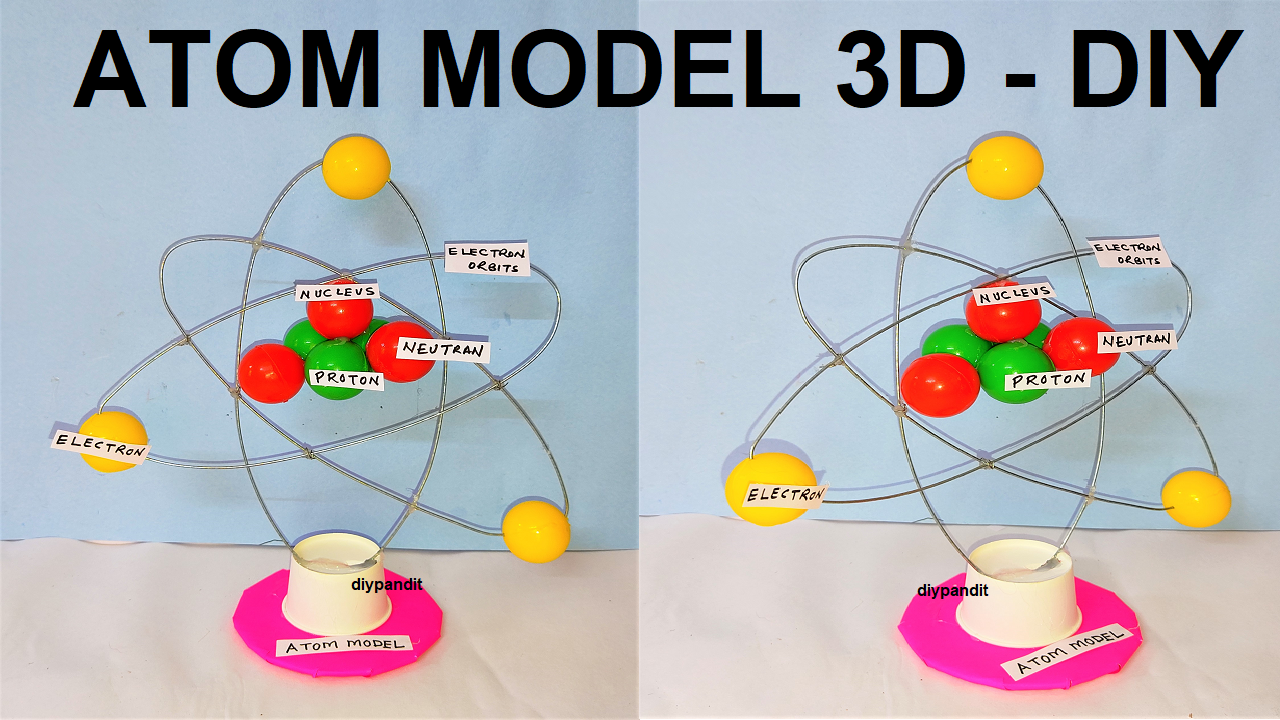
Here are 20 questions and answers related to an atom structure model for a science exhibition:

- What is an atom?
- An atom is the smallest unit of an element that retains the chemical properties of that element. It consists of a nucleus containing protons and neutrons, surrounded by electrons.
- What are the main components of an atom?
- The main components of an atom are protons, neutrons, and electrons.
- What is the structure of an atom?
- An atom consists of a nucleus containing protons and neutrons, surrounded by electrons in orbitals or energy levels.
- What are the three subatomic particles found in an atom?
- Protons, neutrons, and electrons are the three subatomic particles found in an atom.
- What are the charges and relative masses of protons, neutrons, and electrons?
- Protons have a positive charge and a relative mass of approximately 1 atomic mass unit (amu). Neutrons have no charge (neutral) and a relative mass of approximately 1 amu. Electrons have a negative charge and a negligible mass.
- How are protons and neutrons arranged in an atom?
- Protons and neutrons are located in the nucleus of the atom, with protons having a positive charge and neutrons being neutral.
- What is the role of the nucleus in an atom?
- The nucleus contains protons and neutrons and is the central part of the atom. It provides the mass of the atom and is surrounded by electrons.
- How are electrons arranged around the nucleus?
- Electrons are arranged in specific energy levels or shells around the nucleus. These energy levels can hold a specific number of electrons based on their energy.
- What is the significance of electron shells or energy levels in an atom?
- Electron shells determine the chemical behavior and properties of an atom. The number of electrons in the outermost shell determines how an atom interacts with other atoms to form chemical bonds.
- How does the number of protons determine the identity of an atom?
- The number of protons in the nucleus of an atom determines its atomic number and identifies the element.
- What is an atomic number, and how is it related to the number of protons?
- The atomic number of an atom is the number of protons in its nucleus. It is unique to each element and determines its position on the periodic table.
- What is an atomic mass, and how is it calculated?
- Atomic mass is the mass of an atom, which includes the combined mass of protons and neutrons in its nucleus. It is calculated by summing the number of protons and neutrons.
- What is an isotope, and how does it differ from a regular atom?
- Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This results in isotopes having different atomic masses.
- How do atoms bond with each other to form molecules?
- Atoms bond with each other through chemical bonds, which involve the sharing or transfer of electrons between atoms to achieve a stable configuration.
- What are the different types of chemical bonds?
- The different types of chemical bonds include covalent bonds, ionic bonds, and metallic bonds.
- What is the Bohr model of the atom?
- The Bohr model of the atom is a simplified model that depicts electrons orbiting the nucleus in discrete energy levels or shells.
- How does the modern quantum mechanical model of the atom differ from the Bohr model?
- The modern quantum mechanical model of the atom describes electrons as existing in probability clouds or orbitals around the nucleus, rather than in fixed orbits as in the Bohr model.
- What are some real-world applications of understanding the structure of atoms?
- Understanding the structure of atoms is essential in various fields, including chemistry, materials science, biology, and engineering. It is used in drug development, nanotechnology, energy production, and environmental science, among others.
- How do scientists study the structure of atoms experimentally?
- Scientists use various experimental techniques, such as spectroscopy, X-ray crystallography, and particle accelerators, to study the structure of atoms and their behavior.
- What are some recent advancements or discoveries related to atomic structure?
- Recent advancements include the discovery of new elements, advances in atomic imaging techniques, and developments in quantum computing and nanotechnology that rely on understanding atomic behavior.