INTRODUCTION
In this topic, we are going to show you how to build an Atom model for your science project or exhibitions using waste materials available at home
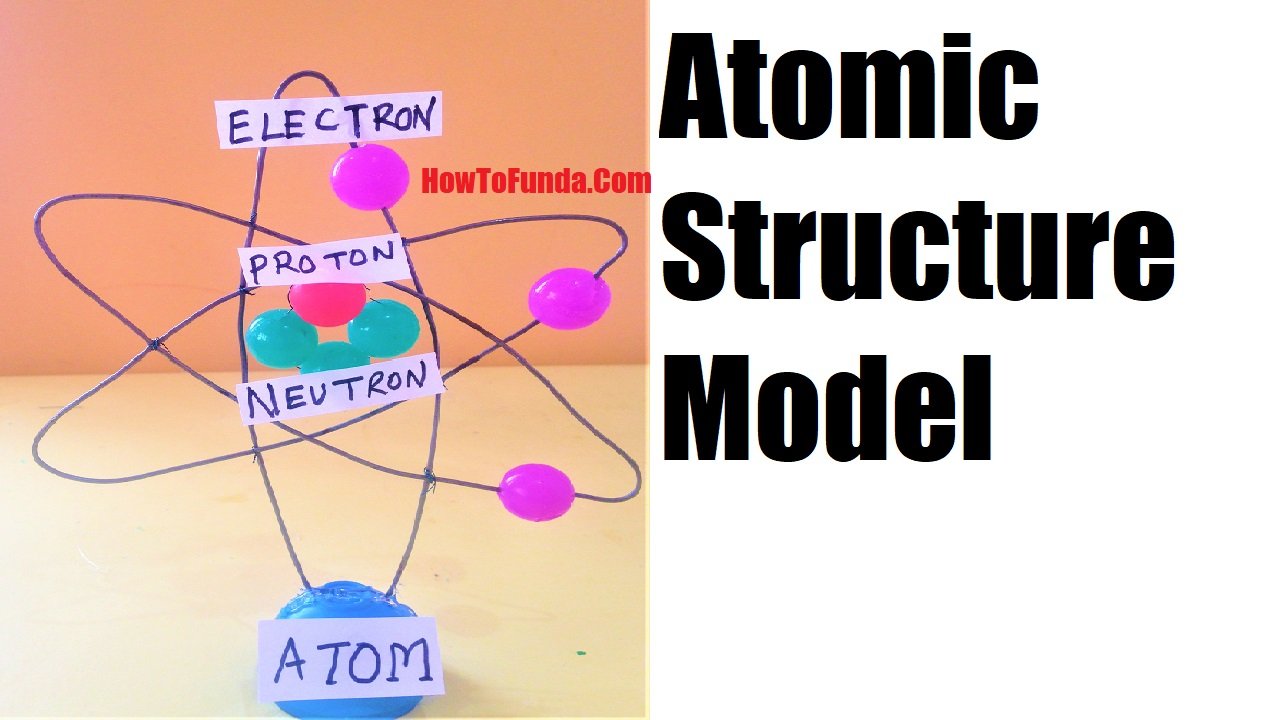
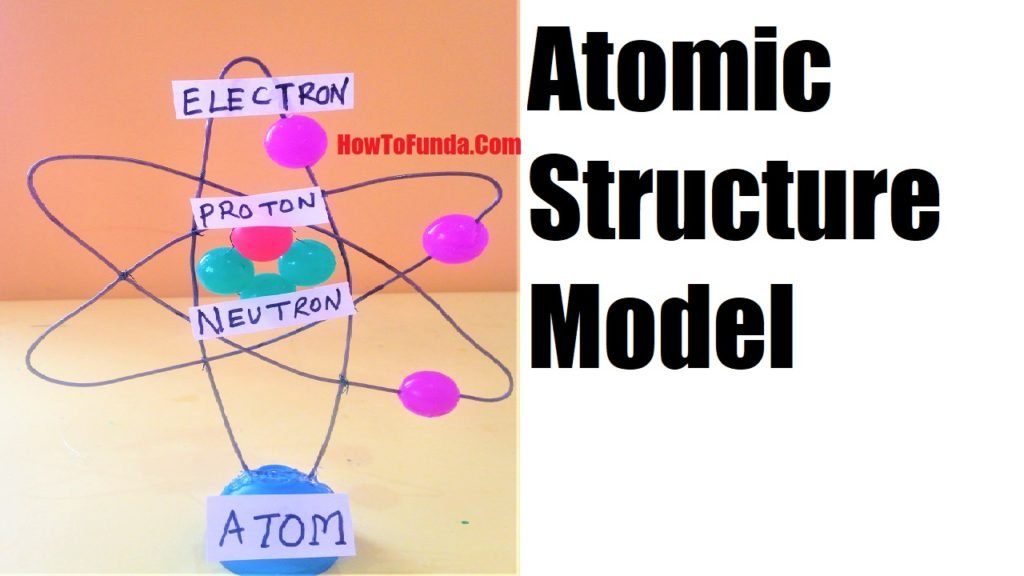
Rutherford–Bohr model depicts a dense nucleus surrounded by revolving electrons which look similar to our solar system and here attraction between electrons is due to electrostatic forces rather than gravity. It was created in 1913.
How To Make an atomic model project (Rutherford Bohr 3D model)
In this video, we will show you steps by step to build an Atom model at home using waste materials available with the help of your parents.
Materials Used to make Rutherford Bohr model
- Small rubber balls
- Toothpicks
- Thick 3 piece GI wires
- Scale
- Thin wire
- Needle
- Blade
- Scissor to Cut
- Hot Glue Gun
Questions & Answers on Bohr’s model
- What does Bohr’s model explain?
Bohr’s model states that electrons in atoms are revolving in orbits which are of different energy around the nucleus. - What is the difference between Rutherford’s model of the atom and Bohr’s model of the atom?
Rutherford stated that atom is a tiny positive mass surrounded by a cloud of negative electrons whereas Bohr’s stated that electron orbited the nucleus in quantized orbits. - Is the Bohr model correct?
Yes, it works very well for only one electron and not at all for multi-electron atoms. - What are the four principles of Bohr’s model?
Bohr’s model consists of principle which states that electrons occupy certain orbits around nucleus and these orbits are called stationary orbit, also every individual orbit has an energy associated with it.
Conclusions
The atomic model helps us to understand how electrons revolve around stationary orbits around the nucleus which was developed by Bohr.