What is Electrolysis of water?
Electrolysis of water is the process of using an electric current to break water molecules (H2O) into hydrogen gas (H2) and oxygen gas (O2) through a process called electrolysis.
This is typically done by passing an electric current through water that has been dissolved with an electrolyte, such as sodium chloride (salt).
The process can be used to produce hydrogen for fuel cells or as a source of oxygen for breathing in underwater situations.
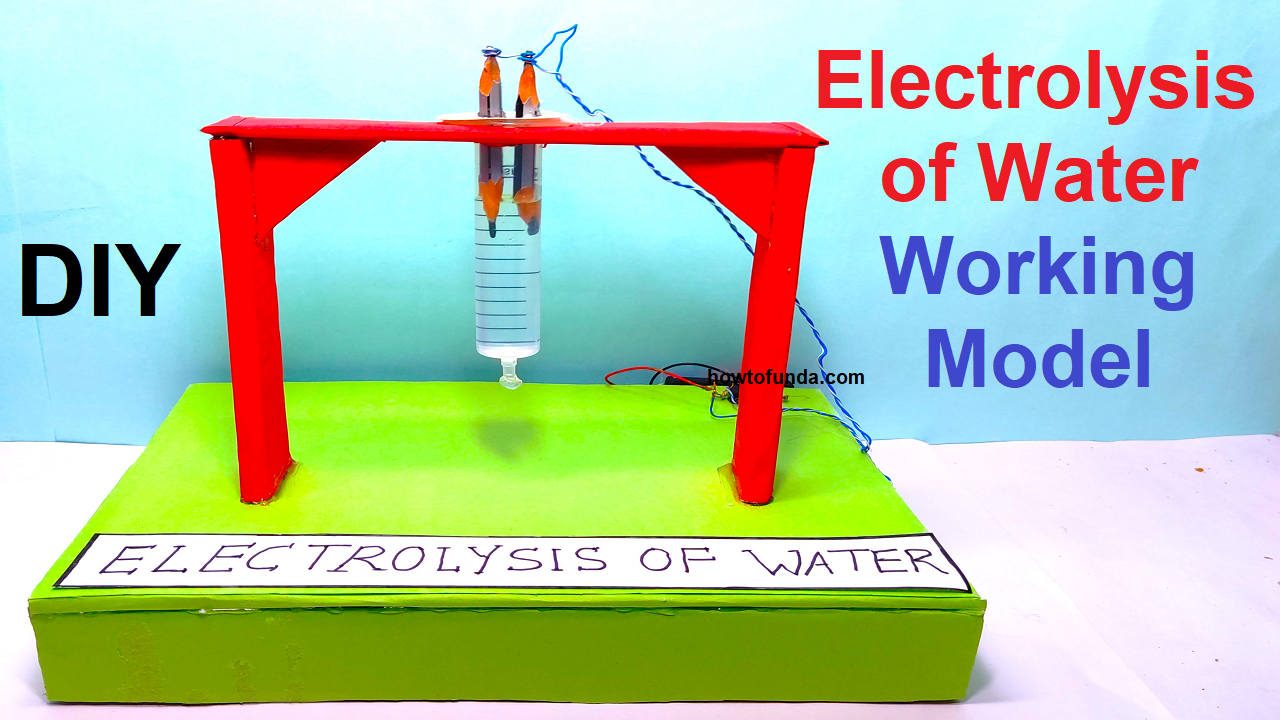
Electrolysis of water working model
An electrolysis of water working model is a device that uses an electrical current to split water molecules (H2O) into hydrogen gas (H2) and oxygen gas (O2).
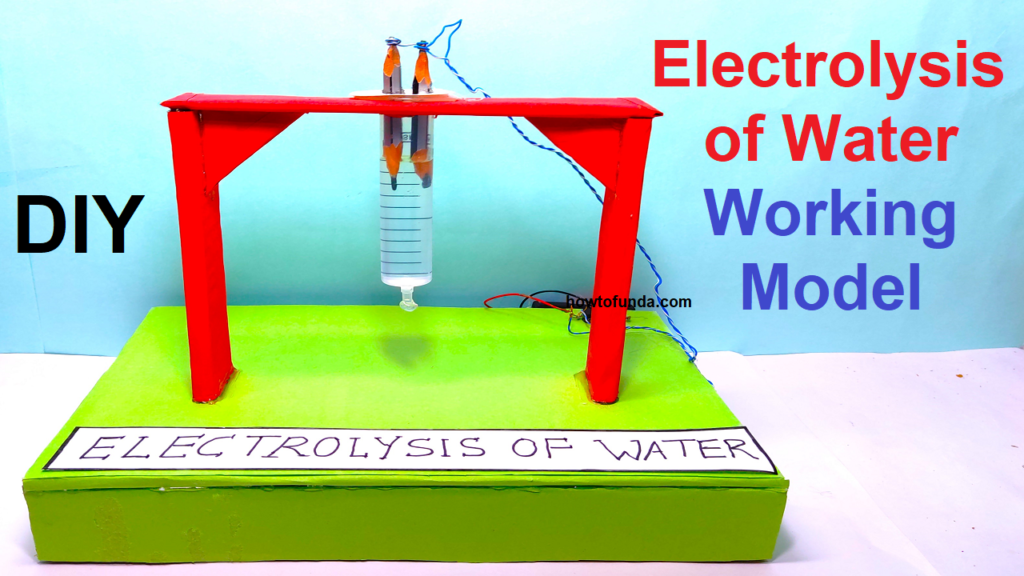
#electrolysisofwater #workingmodel #sciencefair #scienceexhibition #scienceproject #workingproject #chemistrymodel #chemistryproject
Step by Step Video on electrolysis of water working model science project
To make the working model of electrolysis of water typically requires several materials:
- Water: The water serves as the medium in which the electrolysis reaction occurs. It is important to use distilled or deionized water to avoid contamination of the electrodes.
- Electrodes: The electrodes are typically made of a material that is a good conductor of electricity and can also act as a catalyst for the electrolysis reaction. Common materials used for the electrodes include platinum, carbon(pencil), nickel, and iron.
- Power source: The power source is responsible for providing the electrical energy needed for the electrolysis reaction. It can be a battery, a solar cell, or a power supply.
- Wires: The wires are used to connect the electrodes to the power source. They should be made of a material that is a good conductor of electricity, such as copper or aluminum.
- A container: A container is used to hold the water and the electrodes. It can be made of glass, plastic, or any other non-reactive material.
- A switch: A switch is used to turn the power on and off, and to control the flow of electricity through the circuit.
Q: What is electrolysis of water?
A: Electrolysis of water is the process of using an electrical current to split water molecules into hydrogen and oxygen.
This is done by passing an electric current through a water solution that contains an electrolyte, such as salt or acid.
Q: How does the model demonstrate the process of electrolysis of water?
A: The model may use a simple setup consisting of a container of water, electrodes, a power source, and tubing to collect the gases produced. The electrodes are immersed in the water and connected to the power source, and the water is then electrolyzed into hydrogen and oxygen gases, which can be collected in separate tubes.
Q: What are the products of electrolysis of water?
A: The products of electrolysis of water are hydrogen gas (H2) and oxygen gas (O2).
Q: How does the model show the relationship between the voltage and the amount of gas produced?
A: The model may demonstrate how increasing the voltage applied to the electrodes will increase the amount of gas produced, and how reducing the voltage will decrease the amount of gas produced.