Here are 25 working models suitable for 9th-grade chemistry, each designed to illustrate different chemical concepts, processes, and reactions:
- Molecular Models: Construct models of common molecules (e.g., H2O, CO2, CH4) using ball-and-stick kits to show bonding and molecular structure.
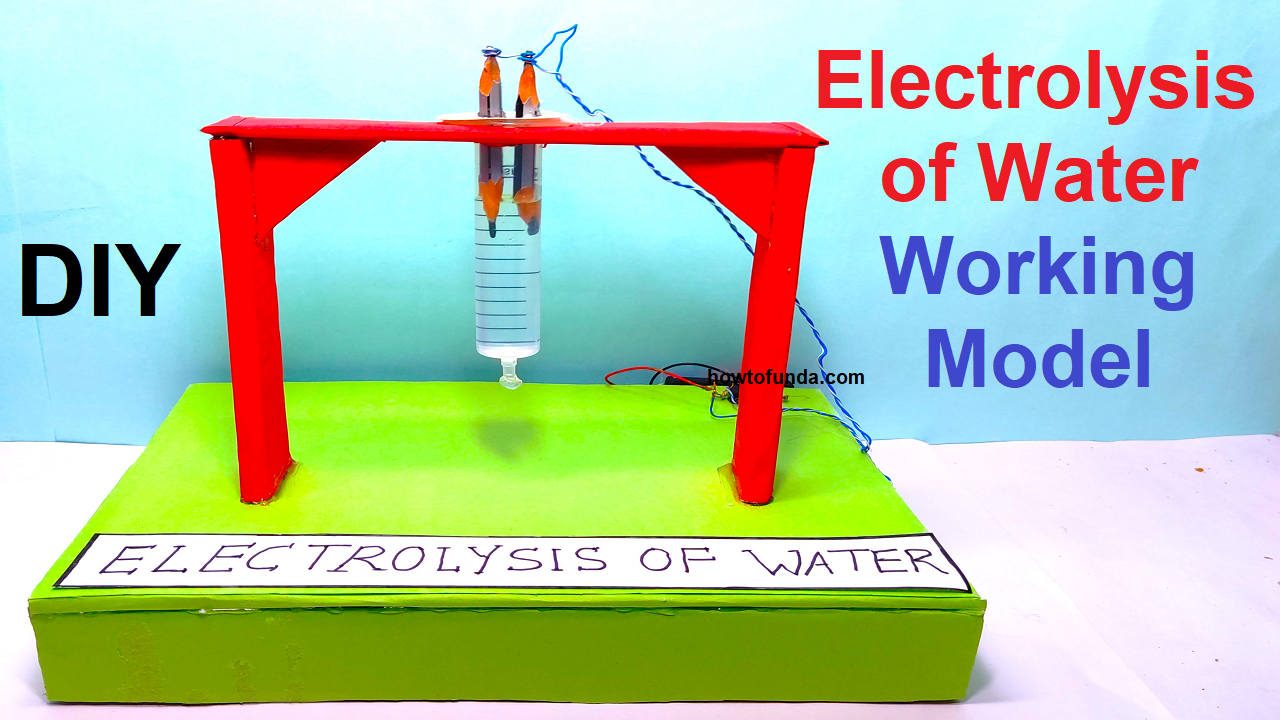
- Electrolysis of Water: Demonstrates the decomposition of water into hydrogen and oxygen gases using a simple electrolysis apparatus.
- pH Indicator with Red Cabbage: Uses red cabbage juice to create a pH indicator, showing color changes in different acidic and basic solutions.
- Volcano Eruption (Chemical Reaction): A model volcano that erupts using baking soda and vinegar, illustrating an acid-base reaction.
- Crystal Growing: Demonstrates crystal formation by growing salt or sugar crystals from a supersaturated solution.
- Periodic Table Display: A visual and interactive model of the periodic table, highlighting element properties and trends.
- Density Column: A layered column of liquids of different densities to illustrate the concept of density and immiscibility.
- Chemical Bonding Models: Using physical models to show ionic, covalent, and metallic bonds.
- Lava Lamp: A homemade lava lamp to demonstrate density and solubility concepts using oil, water, and food coloring.
- Filtration System: A simple filtration model using sand, gravel, and activated charcoal to purify water, demonstrating separation techniques.
- Distillation Apparatus: A basic setup to show the distillation process and separation of mixtures based on boiling points.
- Reacting Metals with Acids: Demonstrates the reaction of metals like zinc or magnesium with hydrochloric acid to produce hydrogen gas.
- Osmosis and Diffusion Model: Uses dialysis tubing to show the principles of osmosis and diffusion with different solute concentrations.
- Exothermic and Endothermic Reactions: Simple experiments to show temperature changes in exothermic and endothermic chemical reactions.
- Soap and Detergent Action: Models illustrating how soaps and detergents work to remove grease and dirt through emulsification.
- Hydrogen Balloon Explosion: Safely demonstrates the explosive reaction of hydrogen gas with oxygen.
- Carbon Dioxide Fire Extinguisher: Shows how carbon dioxide can be used to extinguish a flame, explaining the fire triangle.
- Chromatography: Paper chromatography to separate and identify different pigments in inks or plant extracts.
- Combustion Reaction: A controlled demonstration of a combustion reaction using a small piece of material and a flame.
- Chemical Kinetics: Demonstrates factors affecting reaction rates, such as concentration, temperature, and catalysts, using simple reactions.
- Electroplating: A setup to show the process of electroplating a metal object with copper or another metal.
- Law of Conservation of Mass: Experiments to show that mass is conserved in a closed system during a chemical reaction.
- Gas Laws: Demonstrates Boyle’s Law and Charles’s Law using syringes and balloons to show the relationships between pressure, volume, and temperature.
- Acid-Base Titration: A simple titration experiment using a known concentration of an acid or base to determine the concentration of an unknown solution.
- Slime Making: Demonstrates polymer chemistry by making slime with glue and borax solution, showing cross-linking of polymers.
These working models cover a broad range of fundamental chemistry concepts appropriate for 9th-grade students, providing hands-on experiences to reinforce theoretical knowledge.